News Highlights
The DCGI granted market authorisation to Serum Institute to produce India’s First Cervical Cancer Vaccine Quadrivalent Human Papillomavirus vaccine (qHPV)
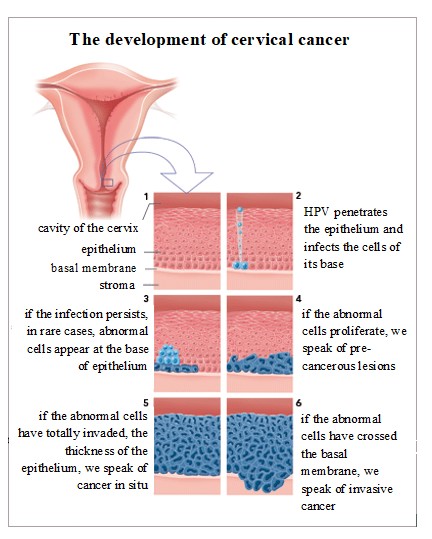
Cervical Cancer
- Cervical cancer develops in a woman’s cervix (the entrance to the uterus from the vagina).
- Various strains of the Human papillomavirus (HPV) play a role in causing most cervical cancer.
- When exposed to HPV, the body’s immune system prevents the virus from harming.
- In a small percentage of people, however, the virus survives for years, contributing to the process that causes some cervical cells to become cancer cells.
What is HPV?
- HPV is a type of virus, of which there are more than 100 types.
- The National Cancer Institute (NCI) notes that more than 40 types of HPV are spread through direct sexual contact.
- Out of these 40 types,
- two cause genital warts
- about a dozen HPV cause different types of cancer including cervical, anal, oropharyngeal, penile, vulvar and vaginal.
- Significantly, almost all cervical cancers are caused by HPV and the vaccine protects against two of the cancer-causing strains, which are HPV 16 and 18.
Types of HPV vaccines
- quadrivalent vaccine (Gardasil): protects against four types of HPV (HPV 16, 18, 6 and 11). The latter two strains cause genital warts.
- Bivalent vaccine (Cervarix): protects against HPV 16 and 18 only.
- Non valent vaccine (Gardasil 9): protects against nine strains of HPV.
Cervical cancer in India
- Cervical cancer in India ranks as the second most frequent cancer among women between 15 and 44 years of age.
- A paper published in the Asian Pacific Journal of Cancer Prevention notes that,
- India is home to 16-17 percent of the world’s population, globally 27 per cent of total cervical cancer cases are from here.
- Further, in India about 77 percent cases of cervical cancer are caused by HPV 16 and 18.
Vaccination in India
- In India, bivalent and quadrivalent HPV vaccines were licensed in 2008 and a non valent vaccine was licensed in 2018.
WHO Cervical Cancer Elimination Strategy Targets for 2030
- 90% of girls fully vaccinated with the HPV vaccine by the age of 15
- 70% of women are screened with a high-performance test by 35 years of age and again by 45 years of age
- 90% of women identified with cervical disease receive treatment
Pic Courtesy: Indian Express
Content Source: Indian Express